Many times, children see plants presented as static objects rather than dynamic, interesting living things. I have a new card set that can help you bring more liveliness and fun to botany. It is called “What Flower Is Growing Here?” The set has close-up photos of buds and on a second card, the flowers that unfold from them. Children look at a bud and see if they can match it to the flower in bloom. There are 16 different species of plants featured, and their study can stimulate bud observations in both spring and fall. You can see the set here. https://big-picture-science.myshopify.com/collections/montessori-botany-materials/products/what-flower-is-growing-here .


There is a sentence or two on the flower card that helps children understand more about the flower. For more advanced children, there is a text card for each plant that gives its classification, more details of its development, and its origin.
Annuals and herbaceous perennials grow most rapidly in spring and summer. They produce their buds and blooms from spring to late summer. Most of them have finished their activities in autumn. Herbaceous plants that are pictured in the “What Flower Is Growing Here?” set include petunias, pansies, poppies, and peonies. Zinnias, hollyhocks, nasturtiums, lilies, and columbines, along with daffodils, daylilies, and the bearded iris, round out the spring and summer bloomers that die back in winter.
In autumn, there are structures to observe in another group of plants, the shrubs and trees. Several woody plants form conspicuous flower buds by autumn and hold them over the winter before they bloom in spring. All of them form leaf buds, and many form flower buds that are hidden in the leaf buds. The woody plants in “What Flower Is Growing Here?” are the rhododendron, flowering dogwood, and star magnolia. All three of these form their flower buds in the late summer or early autumn. They have flower buds that children can easily see all winter long. Other woody plants that form visible flower buds in autumn include alders (shown below), birches, forsythias, and the silktassel (Garrya).

Considering these two categories of plants, there is some flower bud to be seen almost year round. After children have worked with the “What Flower is Growing Here?” cards, they are primed to find buds on nearby plants. They can even look at weeds with a hand lens and may be able to find tiny flower buds there. Following buds through their development is an important activity that helps children see plants as alive and dynamic.
When children see the buds during the winter, they will be primed to observe the big changes that come in spring. If they keep a watch on annuals and perennials in the spring, they may spot the buds well before bloom time. It is exciting and amazing to see what a large flower emerges from some small buds.
The same sort of excitement can come in spring when the leaf buds start to open. Woody plants form their leaf buds in the previous summer or autumn, and most are covered by bud scales. Giving children an opportunity to observe next year’s leaf buds will prepare them to appreciate the swelling bud scales and leaf emergence in the spring.
Enjoy watching your plants bring forth their buds and flowers.
Studies of the diversity of life are a pillar of life science at elementary level. In the past, Montessori classrooms used charts that show Linnaean classification – Kingdom, Phylum, Class, etc. Those charts are no longer very useful except in studies of the history of science. Instead, children need an introduction to the Tree of Life, which they can get via a branching diagram aka family tree, evolutionary tree, phylogenetic diagram, or phylogeny. If you need a Tree of Life diagram, you can download one for free at my website.
In early childhood, children sort pictures under labels, beginning with living vs. nonliving, animal vs. plant, and invertebrate vs. vertebrate, for example. Later, they sort pictures under more categories such as classes of vertebrates or phyla of invertebrates. The activity in my new material, Sorting Branches on the Tree of Life, will look somewhat familiar to children, but it has enough differences to make it challenging and interesting.
As Montessori classrooms adapt to the changing world of academic knowledge, one of the first things will be to help children learn the main branches on the Tree of Life. They need an introduction to the Tree of Life to get an overview, and then they are ready to start studying the main branches. Note that I use the terms “clade,” “lineage,” and “branch” to mean more or less the same thing – an ancestor and all of its descendants.
A challenge of Tree of Life classification is that the big branches have little branches, and the branches are not ranked (aren’t a phylum, class, etc). One simply has to know that the vertebrates are a branch of the chordates, for example. It really isn’t as hard as it sounds. Sorting Branches on the Tree of Life will help children and adults learn the main branches as they sort photos of organisms under a set of heading labels. When children have completed the diagrams, they will be able to see that the organisms belong to a number of clades. They will also be more prepared to use phylogenies (branching diagrams) that show the main branches. Older ones may even want to try their hand at drawing a phylogeny based on a diagram they have completed.
Sorting Branches on the Tree of Life covers the vertebrates and the plants. It has a series of lessons, each of which builds on the last to help children learn the clades (branches, lineages). The lessons use images of extant animals (with one exception) and plants, but they tie into some of the history of the clades as well. One really can’t teach about the diversity of life without giving information about the origin of the branches of life.
I’ll start with descriptions of the lessons for the vertebrates in this article and leave the plants for another day. The first lesson shows the earliest branching of the vertebrates, which produced the jawless fish and the vertebrates with jaws. The latter clade, called the gnathostomes, has two branches, the cartilaginous fishes and the bony vertebrates. I have called the second branch the bony vertebrates instead of the bony fishes because it holds more than just fish. It is actually our branch as well.
The bony vertebrates have two branches, the ray-finned fish and the lobe-fins. The latter includes the coelacanth and the lungfish, as well as the tetrapods, the animals with four limbs. I used a picture of a lion to represent the tetrapods so that children could see that ALL the tetrapods belong to that lineage, not just the amphibian-like, first ones to evolve.

The second lesson shows the branches of the tetrapods, and its diagram shows that birds are a branch of the reptiles.

Reptiles, birds, mammals, and eutherian mammals each have another lesson with a diagram. The reptile and mammal lessons come after children have had the tetrapod lesson. The reptile lesson shows that this branch of life divides into the lepidosaurs (“scaly lizards”) and the archosaurs (“ruling lizards”). For the latter, the branches are the crocodilians (crocodiles, alligators, and relatives) and the dinosaurs. The pictures under the dinosaur label are a non-avian dinosaur and an avian dinosaur – a chicken. Yes, the birds are really dinosaurs, and they should be placed under the archosaur label.
Don’t panic at the idea of birds being a branch of the reptiles. We can still teach about those two branches of life separately. The traditional reptile lessons usually give the characteristics of the squamate reptiles – lizards and snakes – or of turtles, which are a world of their own, a sister branch to the archosaurs. Lessons can emphasize the traits that birds and crocodilians share. Studies of birds can note their reptile-like traits such as scaly skin on their legs.
The mammal diagram shows the first two branches as the monotremes and the therians. This omits a lot of mammal history, but the point of these lessons is not the whole history of the organisms. It is about the branches of the currently living ones. The therians are the marsupials and the eutherian mammals, aka placental animals. When you have finished the lesson on the eutherian mammals, children can go back through and make a list of their own branches of life.
I produced this material this spring, and children in a Montessori classroom got to see a prototype just before the schools closed because of the pandemic. The teacher reported that they were very interested in the material, partly because it doesn’t look like all their other materials. By elementary age, children are ready for variety and challenge. Sorting Branches on the Tree of Life supplies both.
I am happy to answer questions you may have about this material. I supply it as a digital download, a file that you can print for yourself. See
https://big-picture-science.myshopify.com/collections/biology/products/sorting-branches-on-the-tree-of-life-vertebrates-and-plants.
In my last post, I took readers on an imaginary tour of nesting boxes for the plant kingdom. These materials are traditionally called Chinese boxes, but I prefer to use “nesting boxes.” Children explore the structure and major lineages of a kingdom of life with this material. Nesting boxes work well for showing the lineages of the animal kingdom provided the content reflects current knowledge.
Here’s an imaginary tour of nesting boxes for the animal kingdom as it is defined today. I believe firmly that we should be giving children terms that they will see in their further studies, not terms that are historical and that do not appear in modern textbooks.
To start our tour, picture a large red box labeled “Animal Kingdom.” We remove the lid, and inside there is a small box that is labeled “Phylum Porifera, the sponges.” This group was once called the Parazoa, but this term has fallen out of favor, and I recommend these animals be called the sponges. Once thought to be several separate lineages, they are now placed on one lineage, Porifera (“the pore-bearers”).
Along with the little Porifera box, there is a much larger box that takes up most of the animal kingdom box. It is labeled “Eumetazoa, the true animals.” We lift the lid, and inside there are two small boxes labeled “Phylum Ctenophora, the comb jellies” and “Phylum Cnidaria, the stingers.” A large box labeled “Bilateria” takes up most of the remaining space, and it holds the animals with bilateral symmetry.
Cnidarians include the sea anemones, corals, and jellyfish. The comb jellies include sea gooseberries and sea walnuts. These two phyla were previously placed in a single phylum. That phylum, Coelenterata, is obsolete and should not appear in current animal kingdom classification studies. Our small red boxes are labeled “Phylum Cnidaria, the stingers,” and “Phylum Ctenophora, the comb-bearers,” and “Coelenterata” is not here at all.
The big box labeled “Bilateria, animals with bilateral symmetry” contains two boxes, which are labeled Protostomes (“mouth first”) and Deuterostomes (“mouth second”). These names reflect a difference in the development of the fertilized egg in these two lineages. The deuterostome box takes up about 1/3 of the space. We look inside it, and we find two boxes, one labeled “Phylum Echinodermata, the spiny skins,” and the other “Phylum Chordata, the corded ones.” The echinoderm box has the sea urchins, sea stars, and sea cucumbers inside. The chordate box has its three subphyla inside, the lancelets, the tunicates, and the vertebrates. Note that chordates are not the same as vertebrates! I’ve seen them mistakenly equated in Montessori materials. (If you find the term “non-chordate” in your materials, it would be best to change it to “invertebrate.”)
The protostome box has two boxes inside, one labeled “Spiralia” or “Lophotrochozoa” and one labeled “Ecdysozoa.” The Spiralia box has the rotifers, the flatworms, the mollusks, and the annelids (segmented worms). This box also has the name Lophotrochozoa although some biologists use this cumbersome term for only a part of the Spiralia. The term Spiralia could change so check again in a few years to see the current story. The Spiralia are named for the pattern of cells in the early embryos of most species.
“Lophotrochozoa” is still used for the Spiralia lineage in many college textbooks, but this could to change by the time elementary children reach college age. I have adopted “Spiralia” because of biologists’ support for it, and it is easier to spell and say. My book, Kingdoms of Life Connected, still has “Lophotrochozoa” because when I reprinted it last year, the term “Spiralia” was not yet shown in Wikipedia (usually a good source for the latest phylogeny). I hope biologists have settled on the name by the time I print the book again.
The ecdysozoa are the molting animals. They shed their whole outer covering at once. This is the most successful animal lineage in terms of numbers of species and numbers of individuals. The Phylum Arthropoda, the jointed feet, and the Phylum Nematoda, the roundworms, are the two main phyla in this box. Tardigrades and velvet worms could also go here if space allows and if you want to get that level of detail.
If any of your animal kingdom materials include “protozoa,” please remove them and study them with the eukaryotic supergroups (protists). They do not belong in the animal kingdom. If your nesting boxes for animals have protozoa, the best time to change this was about 40 years ago. The second best time is now.
I’ve presented a basic look at the animal kingdom here. If you would like further information on the animal kingdom or the lineages I gave in this article, please see my book, Kingdoms of Life Connected. https://big-picture-science.myshopify.com/collections/biology/products/kingdoms-of-life-connected-second-edition (printed) and https://big-picture-science.myshopify.com/collections/biology/products/kingdoms-of-life-connected-ebook-1 (pdf).

If you want to evaluate an animal kingdom chart, look for the groupings I gave for the nesting boxes. The nematodes should be grouped with the arthropods. The echinoderms should be grouped with the chordates. This is because biologists group organisms according to their shared ancestors, not just how they look. The chart from InPrint for Children places related phyla next to each other. See https://big-picture-science.myshopify.com/collections/biology/products/animal-kingdom-chart.

My photo card set for the animal kingdom - https://big-picture-science.myshopify.com/collections/biology/products/zoology-photo-cards-set-1-major-phyla-of-the-animal-kingdom – gives you high quality images of representative animals across the kingdom. They could be used in or alongside a nesting box material.

Happy explorations of the animal kingdom,
Priscilla
PS. I am putting my reply here to two comments below. I'm sorry I don't have pictures of this imaginary material for you, Gail. I, too, am a visual learner. I think Cindy's idea of referring to the animal kingdom diagram from my Tree of Life chart might help. Yes, the lids on the boxes would be like a node on the evolutionary tree (phylogeny). The reason that there isn't a box for the Radiata is that they don't seem to share a common ancestor other than the one for all animals. If they did share a more recent ancestor, they might still be in Coelenterata. They have a similar organization, although the ctenophores are described as biradially symmetrical. They have a combination of radial and bilateral symmetry. The cnidarians are genuinely radially symmetrical. These two phyla came from separate experiments by early animal life. This is different than the the two phyla shown in the Ecdysozoa. They shared a common ancestor - at least there evidence for this in their genomes.
Thank you for sending your questions and comments. Please feel free to ask further questions.
In the Northern Hemisphere, many schools are beginning their new year. Others around the world are in the last term for their school year. Wherever you are in your yearly cycle, please make time for fact-checking the science materials your children use in their classroom.
By fact-checking, I mean that you read the text and look at the illustrations for the learning materials that children will see. Then you confirm the information with reliable references. This sounds fairly straight-forward, but it is time-consuming, and therefore few people do it.
Fact-checking is absolutely critical because anyone can print materials, whether or not they are familiar with the subject matter. The visual impression and first information that children get from a chart will stick with them, whether it is accurate or not.
Some authors of Montessori materials are conscientious and carefully research their works. The illustrations on this page are the animal and plant kingdom charts from InPrint for Children, a company which always produces quality materials that are beautiful and accurate. Its owner and designer, Carolyn Jones-Spearman, is a perfectionist, and it shows in her work. That is why I partner with InPrint and sell those materials.
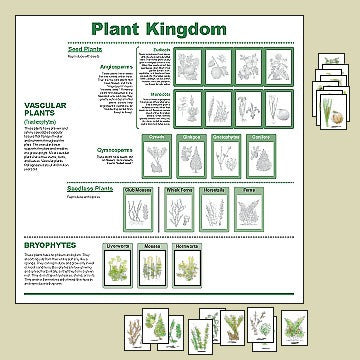

Unfortunately, some authors produce materials with errors or misconceptions because they don’t take time to learn the subject matter or because that is the way they’ve always done it. Some purchase a company and continue to provide its same materials without evaluating them. Certainly, there are commercially available materials that are not suitable as learning materials for children, either because they are outdated, or present false or misleading information.
It appears that all adults who create materials for elementary Montessori children do not have a good grasp of science subject matter. Running a business, printing materials, and marketing them are important skills for a business, and some do that well, even though they are not good at writing or researching valid content. Just because the ads look good, don’t assume that the materials are great.
I suggest that you go over all the materials you will provide to the children, whether those materials are newly printed or older ones that you have in the closet. If the volume is too great for you to cover, perhaps you can get help from older elementary children or secondary students. Children should see fact-checking as a useful activity for anyone.
First, look at the material and its illustrations. Do the illustrations give a clear picture of the subject? Are they indeed examples of the subject? I have seen charts illustrated with organisms that are not the ones being described. I have also seen superficially attractive charts that had artistic but wrong or confusing illustrations. A scientific illustration should clearly depict the features that children need to learn.
Next, read the text. Are there spelling or grammar mistakes? Does the language read smoothly, and is it concise? Most importantly, does the text convey the information clearly? The descriptions on a science chart shouldn’t be a dull list of facts, but they should not be wordy or have convoluted language either. Authors for children need to be held to the same standard of writing as a professional writing for adults. It should be our goal to provide children with examples of good writing in all their materials.
What do you do if you find less than acceptable content in a material? I strongly suggest that you write the publisher or seller of the material and give them a description of the problem. If the content needs to change, as most of biological classification has done in the last 20 years, authors need to know this. Don’t be shy in asking for a corrected version. See how the seller responds. You may wish to return the material and ask for a refund. It shouldn’t matter if you have had the material for a while. If it has serious defects, then you should be able to return it, and you may wish to warn your fellow teachers. Until teachers put pressure on the publishers of Montessori materials to get rid of their mistakes, commercially available products are not likely to improve.
That being said, if you find a simple typo, try putting a white sticker over it and correcting it yourself. The publisher would probably be grateful to have your corrections, but this is not the sort of thing that should cause you to return a material.
I certainly welcome reports of any spelling or grammar mistakes in my works. I seldom get them, however. When I went back through my Plant Lessons book before I printed it last spring, I found a number of grammar mistakes, often having to do with the placement of commas. I’m still learning and striving to improve my writing skills.
If you have specific questions about the contents of a science material, and you have not been able to find the answers on your own, you may email your questions to me. I will try to answer them, although I can’t guarantee how quickly. I'll address finding reliable sources of information in a future blog.
Priscilla
Last June, the organization that officially recognizes the discovery of chemical elements and their names announced the proposed names for the final four elements on the periodic table. This governing body, the International Union of Pure and Applied Chemistry (IUPAC), took suggestions from the discoverers of the elements and then it issued the proposal. People could submit comments about the names for several months, and then in November, the IUPAC published the names. This was the final step in making them official.
The element names and atomic numbers are: nihonium (Nh) for element 113, which is named for the country of Japan; moscovium (Mc) for element 115, named for Moscow, Russia; tennessine (Ts) for element 117, named for the state of Tennessee; and oganesson (Og) for element 118, named after a Russian scientist who helped discover several elements, Yuri Oganessian. A new periodic table with these names is available at the IUPAC website, https://iupac.org/what-we-do/periodic-table-of-elements/ .
So what does this mean for the Montessori classroom? Children are ready for the abstract idea of chemical elements when they are in their elementary years. When they get an introduction to the periodic table, it should include the full set of names. Children should get a least a brief story of how elements get their names and how governing bodies of science fields bring order to science knowledge.
Children need to know, however, that there are elements that one cannot see with one’s eyes. There are quite a number of elements that are known only by the energy, particles, and atoms produced when they undergo radioactive decay.
The image below is from my newly updated card set, Discovering the Periodic Table. It comes with two sets of cards for all 118 elements, one in color and one in black and white. The card on the left is an example of the color set, and in this case sodium's symbol is color-coded red to show it is one of the alkali metals. The other card is the back of the black and white card, and it shows the type of information given for each element - physical properties, chemical properties, and other information. The front of the black and white card is like the card on the left, but with the symbol only outlined.

I updated and expanded Discovering the Periodic Table last summer after the new names were announced. At that time I added some features to help children understand the nature of the largest elements. The elements that cannot be made in visible quantities have symbols with a dotted outline rather than a solid one. The smallest of these is astatine, atomic number 85. Scientists have calculated that if one could make a piece of astatine, it would instantly vaporize itself because of the energy released by its vigorous radioactive decay.
If you tell children this, they may wonder how such an element was ever discovered. If they don’t think of it, help them arrive at this question. We want children to think about what they hear and ask about how we know what we know. The idea to search for astatine came from its place in the periodic table. Mendeleev left a blank beneath iodine on his first periodic table, implying that there was another element in the halogen family. Researchers that first identified this element used a nuclear reactor to bombard bismuth, atomic number 83, with alpha particles. This added two more protons to bismuth nuclei, and produced a small amount of astatine, which quickly decayed. Later, when researchers knew astatine’s characteristics, and they were able to find tiny traces of it in uranium ores.
After astatine, the next element that can’t be made in visible amounts is francium, atomic number 87. The dotted outline symbols don’t show up again until atomic number 101, mendelevium. It and all larger elements cannot be made in visible amounts. Researchers have made so little of elements 104-118 that the chemical properties of these elements are also unknown. In the cards with color-coded symbols from Discovering the Periodic Table, elements 104-118 have gray symbols to show that there is not enough evidence to assign them to a chemical group such the halogens.
Your children may ask if more elements can be discovered. In theory there could be, but if someone does discover more elements, it will be bigger science news than any recent element discovery. Meanwhile, help 6-9 year-olds explore the common everyday elements with the cards set, Elements Around Us from InPrint for Children. The set, Element Knowledge, will help 9-15 year-olds learn element names, symbols, and several significant groups. This set includes the first 111 elements. You can add the names and symbols of the other seven if your children are interested. They certainly won’t see those symbols in any chemical formulas.
The first time I introduced children to the chemical elements, I wanted to give them a sense of where they might find these substances, either as single elements or in combination with others. Laying out the periodic table is one experience with the elements, but it is quite abstract and disconnected with everyday life. I wanted to help children learn about the elements in common substances, items they could encounter and experience.
I made a set of cards that had pictures of items, and I listed the major elements in each one on the back of the card. To let you know how long ago that was, I printed the lists of elements with a dot matrix printer and an Apple II computer. Fast forward a decade or so, and Carolyn Jones of InPrint for Children was designing a new series of materials for study of matter and atoms in Montessori elementary classrooms. We discussed the idea of a card set that shows common objects and their elements. She took the idea and produced an attractive set of cards that she calls “Elements Around Us.” Presently, only Big Picture Science sells this set.

“Elements Around Us” has photos of 20 objects. The set includes two copies of each card, one to leave whole with text that tells the elements, and one to cut apart for matching. We intentionally used some substances to simulate thinking. The photo of a cotton towel (which is mainly cellulose) and table sugar both say “This is composed of carbon, oxygen, and hydrogen.” Cellulose is a macromolecule that is built of sugar molecules. These cards lead to the concept that elements can be joined in many ways to make different substances. The card that shows gold colored coins lists no gold as an ingredient. There are cards for carbon in the form of graphite and of diamond.
After children have worked with the cards, they are often interested in doing more. The “Elements Around Us” set has a black line master called a replicard, which you can copy for children so they can make their own booklets. They can color the outline drawing and write the elements. There are two blanks for children to draw their own object and research its elements.

Elementary children who are past the stage for card materials or who want to pursue the idea further will likely enjoy How to Make a Universe with 92 Ingredients, a book by Adrian Dingle. The book, Planet in a Pebble, by Jan Zalasiewicz, begins with a chapter on the elements in a common beach pebble. This book is for adult general readers, but selections from it can be read to older children or read by secondary students.
Happy element hunting!













